Abstract
Organobismuth compounds, i.e., organic–inorganic hybrid molecules composed of an organic structure and bismuth metal, have been reported to induce cytotoxicity in cancer cells; however, the target proteins associated with this cytotoxicity have not been elucidated. Herein, we investigated the inhibitory effect of five organobismuth compounds on human glyoxalase I (hGLO I), a promising target candidate for cancer therapy. Among these compounds, triphenylbismuth dichloride (Bi-05) exerted a strong inhibitory effect on hGLO I. Indeed, Bi-05 inhibited hGLO I in a dose-dependent manner with an IC50 value of 0.18 µM. Bi-05 also induced cytotoxicity in human leukemia HL-60 cells and human lung cancer NCI-H522 cells, both of which exhibit high expression levels of GLO I. However, the hGLO I-inhibiting and cytotoxic effects of Bi-05 disappeared when the bismuth atom was replaced with an antimony or phosphorus atom. Bismuth(III) nitrate had little inhibitory effect on hGLO I activity and only slightly reduced the viability of cancer cells. In the culture medium of Bi-05–treated HL-60 cells, the concentration of the GLO I substrate methylglyoxal was markedly elevated. In addition, Bi-05 treatment more strongly inhibited human lung cancer NCI-H522 cell (exhibiting high GLO I expression) proliferation than human lung cancer NCI-H460 cell (exhibiting low GLO I expression) proliferation. Furthermore, the cytotoxicity of Bi-05 was significantly decreased by pre- and co-treatment with the methylglyoxal scavengers N-acetyl-L-cysteine and aminoguanidine. Overall, these results suggest that Bi-05 treatment leads to the accumulation of methylglyoxal via GLO I inhibition, resulting in cytotoxic effects in cancer cells.
INTRODUCTION
Organobismuth compounds are organic–inorganic hybrid molecules composed of an organic structure and bismuth metal. These compounds have been shown to induce cytotoxicity in cancer cells. For example, Iuchi et al. (2008) found that 12-chloro-5H-7,12-dihydrodibenz [c,f][1,5] thiabismocine, a heterocyclic organobismuth(III), inhibited the growth of the human promyelocytic leukemia cell line HL-60 and induced apoptosis in HL-60 cells via a caspase-3–mediated mechanism through the generation of reactive oxygen species (ROS) and the release of cytochrome c from mitochondria. Iuchi et al. (2009) also found that bi-chlorodibenzo[c,f][1,5]thiabismocine (compound 3) caused cell cycle arrest at the mitotic phase and induced apoptosis in the human cervical cancer cell line HeLa via an interaction with the colchicine-binding site of tubulin through the SH groups of its cysteine residues. In addition, several other compounds, including 1-[(2-di-p-tolylbismuthanophenyl)diazenyl]pyrrolidine (Onishi et al., 2012), bis-(2-acetylbenzoato)triphenylbismuth(V) (Islam et al., 2016), and 5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl nitrate (Liu et al., 2017), have been reported to induce apoptosis in cultured cancer cells. However, in relation to the cytotoxicity of such compounds, their proteins targets have not been elucidated.
Inorganic bismuth compounds are widely used for the treatment of gastric ulcers and Helicobacter pylori infections. A study on the mechanism underlying the action of inorganic bismuth compounds revealed that bismuth(III) inhibits jack bean urease, a nickel metalloenzyme, by binding to the cysteine residue of the active site (Zhang et al., 2006). Additionally, the organobismuth compounds triphenylbismuth dichloride and tris(4-fluorophenyl)bismuth dichloride have been reported to inhibit jack bean urease (Murafuji et al., 2006).
Glyoxalase I (GLO I), a zinc metalloenzyme, is a promising target candidate for cancer therapy. It catalyzes the glutathione-mediated detoxification of methylglyoxal, one of the side products of tumor-specific aerobic glycolysis (i.e., the Warburg effect) (Thornalley, 1990). Importantly, methylglyoxal has been shown to induce apoptosis in tumor cells (Kang et al., 1996). The abnormal expression and increased activity of GLO I have been reported in many human tumors, including colon (Ranganathan and Tew, 1993), pancreatic (Wang et al., 2012), stomach (Cheng et al., 2012), prostate (Burdelski et al., 2017), breast (Tamori et al., 2018), and lung (Sakamoto et al., 2001) cancers, as well as in melanoma (Bair et al., 2010) and anticancer drug-resistant human leukemia cells (Sakamoto et al., 2000). Therefore, the overexpression of GLO I is considered to be closely associated with carcinogenesis and anticancer drug resistance, and specific inhibitors of GLO I have been investigated as anticancer drugs that can selectively kill GLO I-overexpressing and anticancer drug-resistant tumors. Indeed, we have identified several plant-derived natural compounds, including myricetin (Takasawa et al., 2008), delphinidin (Takasawa et al., 2010), and piceatannol (Takasawa et al., 2017), as human GLO I (hGLO I) inhibitors. Moreover, we have identified a new type of hGLO I-inhibiting compound, namely TLSC702, which has a unique benzothiazole ring with a carboxyl group (Takasawa et al., 2011).
In the present study, we investigated the inhibitory effect of five organobismuth compounds on hGLO I, finding that triphenylbismuth dichloride (Bi-05) exerted a strong inhibitory effect on hGLO I. Furthermore, Bi-05 induced cytotoxicity in human leukemia HL-60 cells and human lung cancer NCI-H522 cells, both of which exhibit the high expression of GLO I. We also compared the hGLO I-inhibiting and cytotoxic effects of Bi-05 with those of compounds containing antimony or phosphorus in place of bismuth.
MATERIALS AND METHODS
Materials
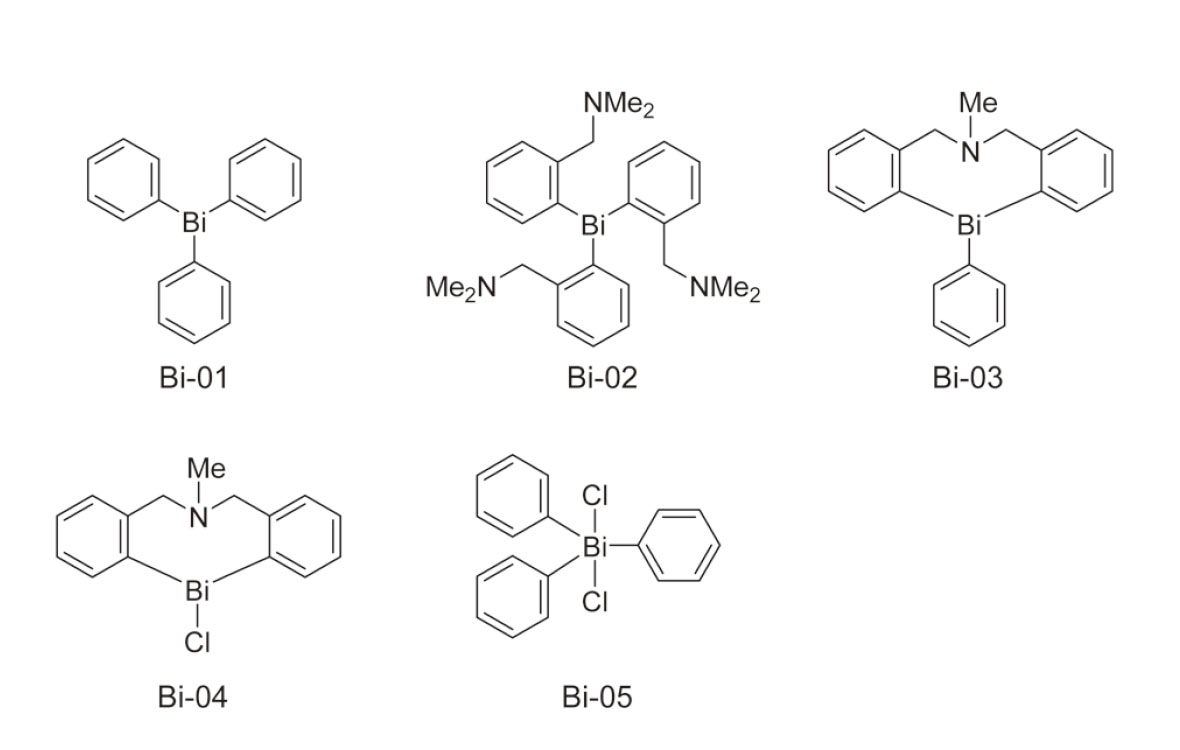
Tris{2-[(dimethylamino)methyl]phenyl}bismuthine (Bi-02; Kamepalli et al., 1996), 12-phenyl-N-methyl-5,6,7,12-tetrahydrodibenz[c,f][1,5]azabismocine (Bi-03; Ohtaka et al., 1989), and 12-chloro-N-methyl-5,6,7,12-tetrahydrodibenz[c,f][1,5]azabismocine (Bi-04; Ohtaka et al., 1989) were synthesized as reported previously. Triphenylbismuthine (Bi-01), Bi-05, triphenylantimony dichloride (Sb-05), o-phenylene diamine (o-PD), 5-methylquinoxaline (5-MQ), and aminoguanidine hydrochloride (AG) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Bismuth(III) nitrate and N-acetyl-L-cysteine (NAC) were obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Triphenylphosphine dichloride (P-05) and 2-methylquinoxaline (2-MQ) were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). S-p-bromobenzylglutathione cyclopentyl diester (BBGC) was provided as a gift by Taiho Pharmaceutical Co., Ltd (Tokyo, Japan). All other chemicals used were of reagent grade.
Expression and purification of recombinant His-tagged GLO I protein in an Escherichia coli expression system
A hGLO I cDNA fragment was subcloned into pET-28a(+) (Merck KGaA, Darmstadt, Germany), and the resulting construct was transformed into BL21(DE3) to produce recombinant hGLO I (rhGLO I) as a hexahistidine fusion protein. Subsequently, rhGLO I protein was purified using TALON Metal Affinity Resins (Takara Bio Inc., Shiga, Japan) in the native condition following the manufacturer’s protocol.
In vitro GLO I assay
A GLO I assay was performed using a spectrophotometric method to monitor the increase in absorbance at 240 nm due to the formation of S-D-lactoylglutathione for 5 min at 25°C. (Takasawa et al., 2008) The standard assay mixture contained 7.9 mM methylglyoxal, 1 mM glutathione, 14.6 mM magnesium sulfate, and 182 mM imidazole–HCl (pH 7.0). Before the reaction was initiated by adding rhGLO I to the assay mixture, the mixture was allowed to stand for 15 min to ensure the equilibration of hemithioacetal formation. The rhGLO I (20 ng) was preincubated with 10 μM of the organobismuth compounds (Bi-01 to Bi-05), bismuth(III) nitrate, Sb-05, and P-05 or with various concentrations of Bi-05 (0.01, 0.03, 0.1, 0.3, 1, 3, and 10 μM) for 10 min, after which it was added to the assay mixture. Absorbance at 240 nm was read in kinetic mode for 5 min.
Cell culture
Cells of the human myeloid leukemia cell line HL-60 and two human non-small cell lung cancer cell lines, NCI-H522 and NCI-H460, were maintained in RPMI1640 supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% fetal bovine serum. The cells were grown at 37°C in a humidified atmosphere containing 5% CO2.
Measurements of cell viability
HL-60, NCI-H522, and NCI-H460 cells were seeded into 96-well plates and cultured for 24 hr. HL-60 cells were treated with 1, 2, and 4 µM Bi-05, Sb-05, P-05, or bismuth(III) nitrite for 48 hr. NCI-H522 cells were treated with 1, 2, and 5 µM Bi-05, Sb-05, P-05, or bismuth(III) nitrite for 72 hr. NCI-H460 cells were treated with 1, 2, and 5 µM Bi-05 for 72 hr. To investigate the effects of NAC and AG on Bi-05–induced cytotoxicity, NCI-H522 cells were pretreated with 600 µM NAC or 100 µM AG for 30 min and then cotreated with 1, 2, and 5 µM Bi-05 and 600 µM NAC or 100 µM AG for 48 hr. Cell viability was detected via a WST-8 assay using a Cell Counting Kit-8 according to the manufacturer’s instructions (Dojindo Laboratories, Kumamoto, Japan). The absorbance of the formazan dye formed was measured at a wavelength of 450 nm using a SpectraMax Microplate Reader (Molecular Devices, San Jose, CA, USA).
Determination of the methylglyoxal concentration of the culture medium
Methylglyoxal concentrations in the culture medium of HL-60 cells were measured according to the method of Hikita et al. (2015) based on the derivatization of methylglyoxal with o-PD and the measurement of the resulting 2-MQ with HPLC/UV. HL-60 cells were seeded at a density of 3.0 × 106 cells in a 6 cm dish and then treated with 4 µM Bi-05, Sb-05, P-05, and bismuth(III) nitrate as the test compounds or 15 µM BBGC as the positive control, after which they were further incubated for 24 hr in a CO2 incubator. The culture medium was obtained via centrifugation at 1,300 rpm for 10 min, and 4.5 mL aliquots were deproteinized with perchloric acid. The supernatant was then obtained via centrifugation at 8,000 rpm for 10 min and applied to a Sep-Pak Light tC18 cartridge (Waters Corporation, Milford, MA, USA). The flow-through fraction was collected and supplemented with 125 nmol o-PD and 12.5 nmol 5-MQ as an internal standard. After standing for 3.5 hr at room temperature, the reaction mixture was applied to another Sep-Pak Light tC18 cartridge. After washing with 10 mM KH2PO4 (pH 2.5), 2-MQ and 5-MQ were eluted using 2 mL of acetonitrile. The eluate was evaporated to dryness using nitrogen blowing, and the residue was reconstituted in 100 µL of the mobile phase, after which 20 µL of each sample was injected in the HPLC instrument. The amounts of 2-MQ and 5-MQ were measured using an Extrema HPLC system (JASCO Corporation, Tokyo, Japan) equipped with a UV-4075 UV/VIS detector (wavelength: 315 nm; JASCO Corporation). The column used was the reversed phase TSKgel ODS-80Ts (4.6 × 250, 5 µm; Tosoh Corporation, Tokyo, Japan), and the mobile phase was 68 vol% of 10 mM KH2PO4 (pH 2.5) and 32 vol% of acetonitrile.
Statistical analysis
Data are presented as means ± standard errors (SEs). Statistical analyses were performed using Microsoft Excel. Significant differences between two groups were evaluated using Student’s t-test with statistical significance set at p < 0.05.
RESULTS
In vitro hGLO I-inhibiting effects of organobismuth compounds
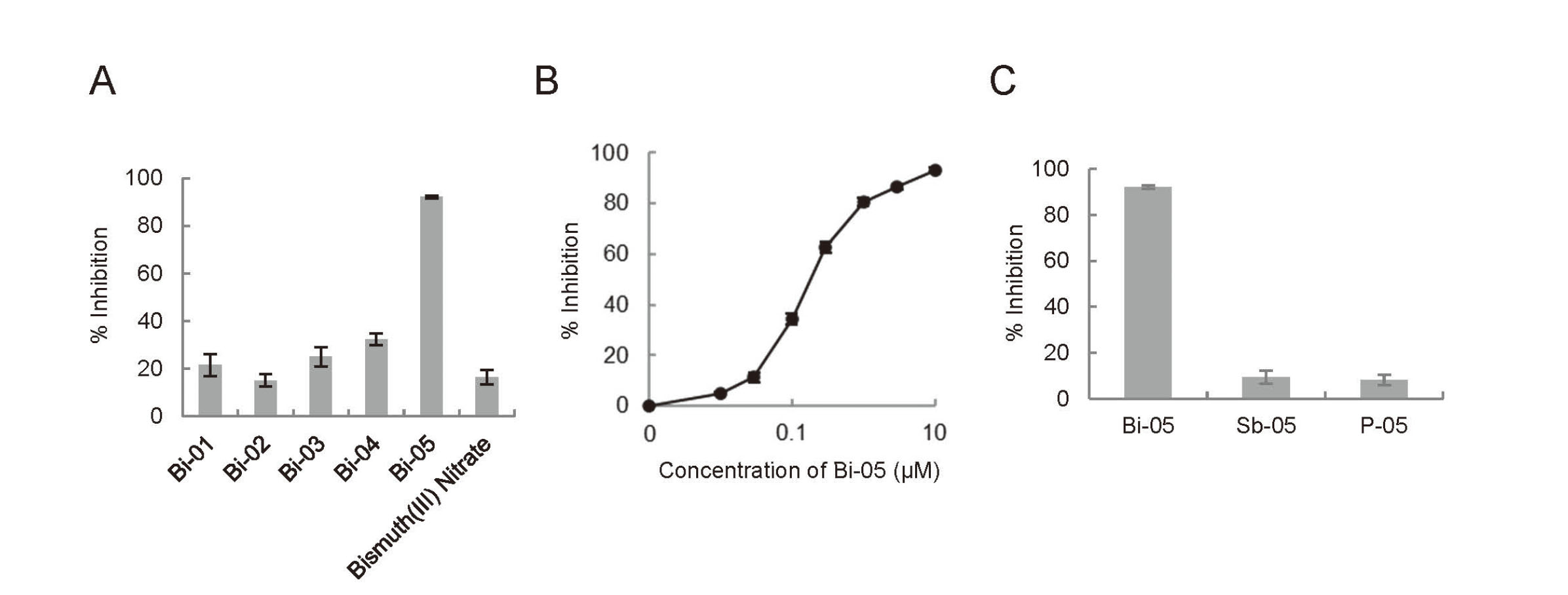
The structures of the five organobismuth compounds used in the present study, Bi-01 to Bi-05, are shown in Fig. 1. First, the inhibitory effects of these organobismuth compounds on hGLO I activity were evaluated. Bi-05 exerted a strong inhibitory effect on hGLO I activity, whereas bismuth(III) nitrate, an inorganic bismuth salt, exerted little inhibitory effect on hGLO I activity (Fig. 2A). As shown in Fig. 2B, Bi-05 inhibited hGLO I activity in a dose-dependent manner and had an IC50 value of 0.18 µM. Unlike Bi-05, compounds containing antimony (Sb-05) or phosphorus (P-05) in place of bismuth did not inhibit hGLO I activity (Fig. 2C).
Antiproliferative effects of Bi-05 and its counterparts on HL-60 cells
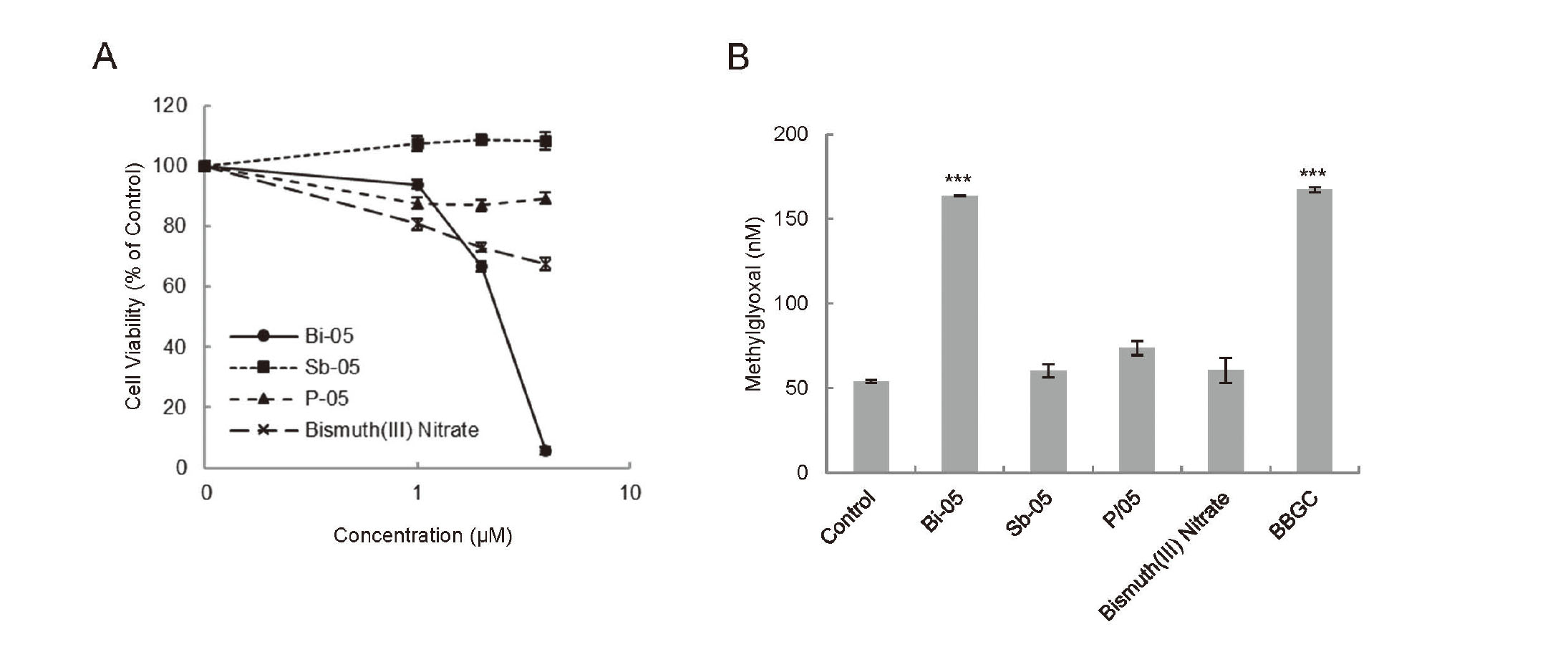
GLO I inhibitors, including the glutathione analog compound BBGC (Thornalley et al., 1996), myricetin (Takasawa et al., 2008), delphinidin (Takasawa et al., 2010), piceatannol (Takasawa et al., 2017), and TLSC702 (Takasawa et al., 2016), have been reported to induce antiproliferative effects in HL-60 cells. Thus, we investigated the antiproliferative effects of Bi-05 and its counterparts Sb-05, P-05, and bismuth(III) nitrite on HL-60 cells. Bi-05 treatment for 48 hr suppressed the proliferation of HL-60 cells in a dose-dependent manner (Fig. 3A), with the EC50 value calculated as 2.4 µM. In contrast, Sb-05 and P-05 did not affect the proliferation of HL-60 cells, and bismuth(III) nitrite only slightly decreased the proliferation of these cells (Fig. 3A).
Methylglyoxal accumulation in the culture medium of HL-60 cells treated with Bi-05
The methylglyoxal concentration in the culture medium of HL-60 cells was significantly increased when the cells were treated with GLO I inhibitors such as BBCG and TLSC702 (Azuma et al., 2021). Therefore, we measured the methylglyoxal concentration of the culture medium of Bi-05–, Sb-05–, P-05–, or bismuth(III) nitrite–treated HL-60 cells as well as BBGC-treated HL-60 cells as the positive control. The methylglyoxal concentrations in the culture medium of HL-60 cells treated with 4 μM Bi-05 and 15 μM BBGC for 24 hr were approximately 3-fold higher than that of the control cells, whereas treatment with 4 μM Sb-05, P-05, or bismuth(III) nitrite did not affect the methylglyoxal concentration in the culture medium of HL-60 cells (Fig. 3B).
Antiproliferative effects of Bi-05 and its counterparts on lung cancer cells
We also evaluated the antiproliferative effects of Bi-05 on the human non-small cell lung cancer cell line NCI-H522 because these cells exhibit high expression levels of GLO I (Takasawa et al., 2016) and the suppression of GLO I with GLO I inhibitors (Takasawa et al., 2016, 2017) or the RNAi knockdown of GLO I (Santarius et al., 2010) has been shown to significantly reduce the proliferation of these cells. Treatment with Bi-05 for 72 hr decreased the proliferation of NCI-H522 cells in a dose-dependent manner, with the EC50 value calculated as 1.0 µM. In contrast, Sb-05 and P-05 treatments did not affect the proliferation of NCI-H522 cells, and bismuth(III) nitrite only slightly decreased the proliferation of these cells (Fig. 4A).
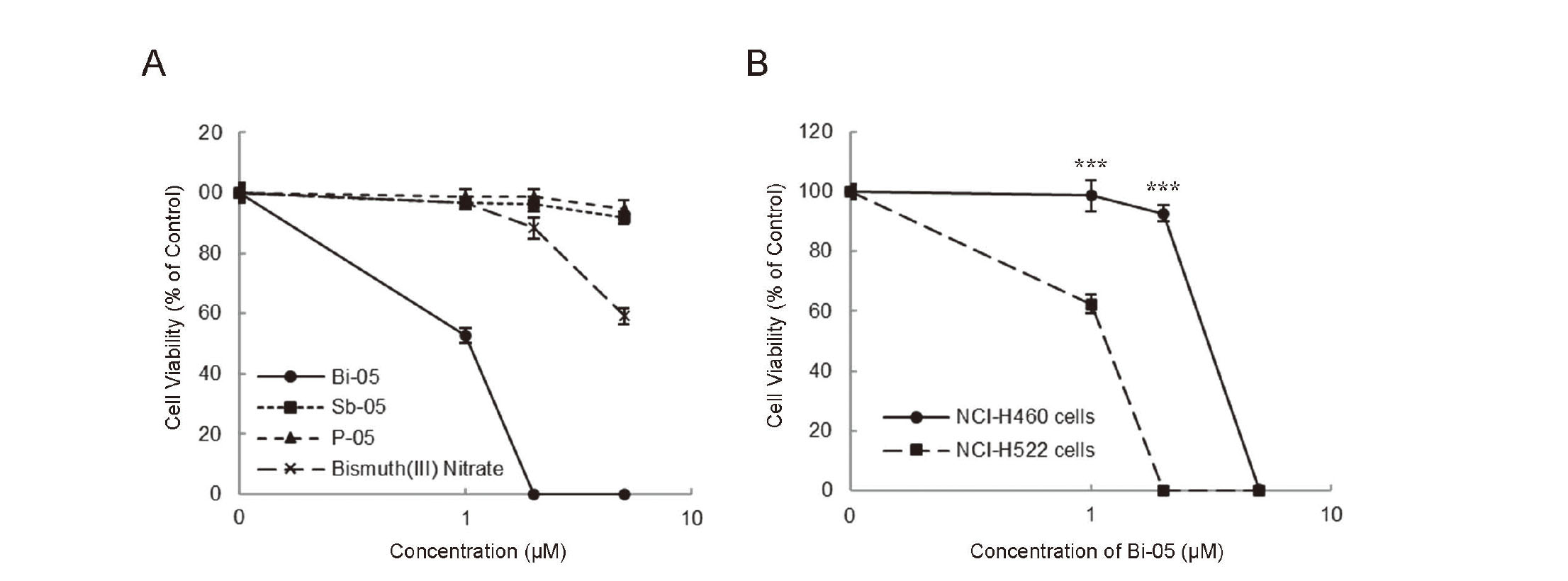
BBGC has been reported to more strongly suppress human lung cancer NCI-H522 cell (with high GLO I activity) proliferation than human lung cancer NCI-H460 cell (with low GLO I activity) proliferation (Sakamoto et al., 2001). We have also shown that TLSC702 and piceatannol more strongly inhibit NCI-H522 cell proliferation than NCI-H460 cell proliferation (Takasawa et al., 2016, 2017). In the present study, Bi-05 preferentially suppressed the proliferation of NCI-H522 cells relative to NCI-H460 cells. The EC50 values of Bi-05 for NCI-H522 and NCI-H460 cells at 72 hr of treatment were 1.0 and 3.1 μM, respectively.
Effects of methylglyoxal scavengers on Bi-05–induced cytotoxicity in NCI-H522 cells
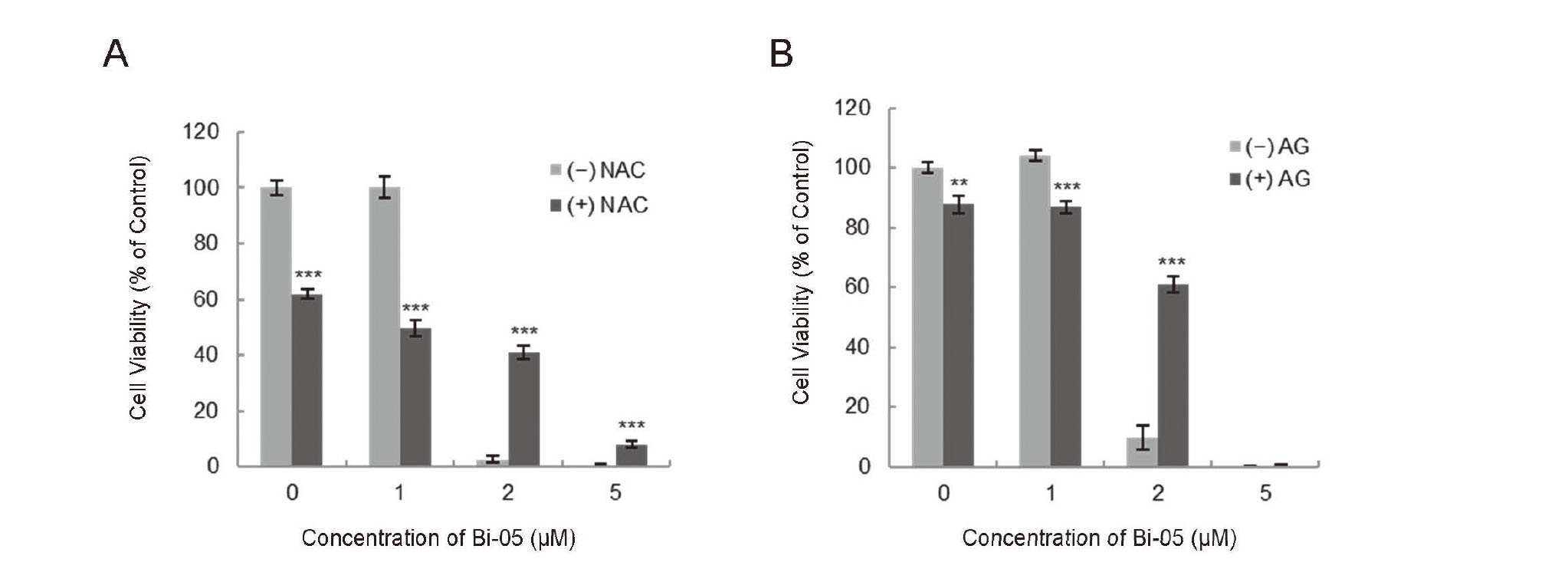
Previously, we reported that the inhibition of GLO I led to the accumulation of methylglyoxal as an argpyrimidine adduct in NCI-H522 cells (Takasawa et al., 2016). Because methylglyoxal-mediated cytotoxicity can be attenuated through treatment with methylglyoxal scavengers such as NAC and AG (Lee et al., 2020), we investigated the effects of these methylglyoxal scavengers on Bi-05–induced cytotoxicity in NCI-H522 cells. As shown in Fig. 5, although 600 µM NAC alone decreased cell viability by about 40%, pre- and co-treatment with 600 µM NAC significantly attenuated the cytotoxic effects of Bi-05 at 2 and 5 µM. Furthermore, pre- and co-treatment with 100 µM AG significantly diminished the cytotoxic effects of Bi-05 at 2 µM.
DISCUSSION
In the present study, we found that Bi-05 exerted a strong inhibitory effect on hGLO I activity. The IC50 value of Bi-05 was 0.18 µM, whereas the IC50 values of other hGLO I inhibitors, namely myricetin, delphinidin, piceatannol, and TLSC702, were 0.56, 1.9, 0.76, and 2.0 µM, respectively (Takasawa et al., 2008, 2010, 2011, 2017), making Bi-05 the strongest inhibitor of hGLO I those tested. Many other natural or natural product-based GLO I inhibitors, glutathione-based GLO I inhibitors, and other types of GLO I inhibitor have been reported, some of which exhibit stronger inhibitory activity than Bi-05 (Jin et al., 2020); however, to the best of our knowledge, the inhibitory effects of organic–inorganic hybrid molecules on GLO I have not been studied previously. Thus, our finding that an organic–inorganic hybrid molecule inhibits GLO I, which is already an attractive target candidate for cancer therapy, is important.
Among the organobismuth compounds evaluated in the current study, only the organobismuth(V) compound Bi-05 exhibited hGLO I-inhibiting activity, whereas organobismuth(III) compounds did not exhibit such activity. Both inorganic bismuth(III) (Zhang et al., 2006) and Bi-05 (Murafuji et al., 2006) have previously been shown to inhibit jack bean urease. In the present study, bismuth(III) nitrate exerted little inhibitory effect on hGLO I activity. Therefore, both the organic structure and bismuth atom of Bi-05 are apparently required for hGLO I inhibition. Bismuth(III) nitrate (Zhang et al., 2006) and organobismuth(III) compound 3 (Iuchi et al., 2009) have been reported to bind to the SH groups of cysteine residues in the active sites of target proteins. So, Bi-05, which has the Lewis acidic bismuth center, is also expected to bind to the SH groups of the cysteine residue involved in the activity of GLO I. Cameron et al. (1997) determined the crystal structure of the dimeric human GLO I enzyme in complex with a glutathione analog inhibitor, S-benzyl-glutathione. The active site zinc ion is coordinated by four amino residues of GLO I (i.e., Gln 33A, Glu 99A, His 126B, and Glu 172B) and one water molecule. At the inhibitor binding site, the hydrophobic binding cavity is lined by hydrophobic residues including the cysteine residue Cys 60A. However, there is no cysteine residue in the active site of hGLO I. Thus, further studies, including the determination of the crystal structure of the hGLO I/Bi-05 complex, are required to elucidate the binding mode of Bi-05 to hGLO I.
Our data in this study suggested that the entire bismuth-containing structure of the organic–inorganic hybrid molecule is necessary for the GLO I-inhibiting activity and cytotoxicity of Bi-05. Studies have shown that organobismuth(III) compounds, such as 1-[(2-di-p-tolylbismuthanophenyl)diazenyl]pyrrolidine, 2-(N,N-dimethylaminomethyl)phenylbis(4-methylphenyl)bismuthane, Bi-phenyl-N-methyl-5,6,7,12-tetrahydrodibenz[c,f][1,5]azabisimocine, accumulate extensively within cells and exert cytotoxic effects, whereas organoantimony compounds with corresponding organic structures accumulate to a lesser extent and are not cytotoxic (Onishi et al., 2012; Kohri et al., 2015). Further studies on the accumulation of Bi-05 and Sb-05 in cells are needed to clarify whether the difference in the hGLO I-inhibiting effects of Bi-05 and Sb-05 contributes to differences in their cytotoxicity. Furthermore, Bi-05 more strongly inhibits human lung cancer NCI-H522 cell proliferation than human lung cancer NCI-H460 cell proliferation, with these cells exhibiting high and low GLO I expression levels, respectively, suggesting that the organobismuth compound Bi-05 exerts potent cytotoxicity preferentially in GLO I highly expressing and GLO I-dependent cancer cells.
At a dose of 2 µM, the cytotoxicity of Bi-05 in NCI-H522 cells was significantly attenuated by treatment with the methylglyoxal scavengers NAC and AG, suggesting that Bi-05 inhibits hGLO I activity, leading to methylglyoxal accumulation and cytotoxic effects. In addition, NAC but not AG partially restored the viability of 5 µM Bi-05–treated NCI-H522 cells. NAC is not only a methylglyoxal scavenger but also exerts antioxidant activity thorough direct effects (e.g., anti-ROS effects, anti-electrophile effects, and the inactivation of metabolites) and indirect effects (e.g., the production of glutathione and hydropersulfides) (Zhitkovich, 2019). The pretreatment of the cells with NAC has been shown to suppress apoptosis induced by treatment with the organobismuth(III) compounds 12-chloro-5H-7,12-dihydrodibenz [c,f][1,5] thiabismocine (Iuchi et al., 2008) and 1-[(2-di-p-tolylbismuthanophenyl)diazenyl]pyrrolidine (Onishi et al., 2012). Moreover, these organobismuth(III) compounds are shown to generate intracellular ROS, reduce mitochondrial membrane potential, and induce apoptosis in leukemia cells. (Iuchi et al., 2008; Onishi et al., 2012) Thus, it may be possible that Bi-05 treatment induces ROS production as well as inhibiting hGLO I activity.
In summary, this is the first study to reveal that an organobismuth compound, Bi-05, inhibits hGLO I activity and induces cytotoxicity in GLO I-dependent tumor cells. Therefore, Bi-05 might represent a novel seed compound for generating new leads for anticancer drugs targeting GLO I. Moreover, organic–inorganic hybrid molecules might be generally useful for developing novel hGLO I inhibitors for use in cancer therapy. We demonstrated previously that hGLO I inhibitors induce apoptosis in GLO I-dependent tumor cells (Takasawa et al., 2010, 2011, 2017). However, further studies are needed to determine whether the cytotoxicity of Bi-05 is due to the induction of apoptosis or other types of cell death. To this end, we are currently examining the hallmarks of apoptosis, i.e., apoptotic morphological changes, nucleosomal DNA fragmentation, and the activation of caspases. Such investigations will likely contribute to the application of organobismuth compounds as cancer therapeutics targeting GLO I.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid for Scientific Research(C) from Japan Society for the Promotion of Science (to R.T., JSPS KAKENHI Grant Number JP19K05737).
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Azuma, M., Inoue, M., Nishida, A., Akahane, H., Kitajima, M., Natani, S., Chimori, R., Yoshimori, A., Mano, Y., Uchiro, H., Tanuma, S.I. and Takasawa, R. (2021): Addition of hydrophobic side chains improve the apoptosis inducibility of the human glyoxalase I inhibitor, TLSC702. Bioorg. Med. Chem. Lett., 40, 127918.
- Bair, W.B. 3rd, Cabello, C.M., Uchida, K., Bause, A.S. and Wondrak, G.T. (2010): GLO1 overexpression in human malignant melanoma. Melanoma Res., 20, 85-96.
- Burdelski, C., Shihada, R., Hinsch, A., Angerer, A., Göbel, C., Friedrich, E., Hube-Magg, C., Burdak-Rothkamm, S., Kluth, M., Simon, R., Möller-Koop, C., Sauter, G., Büscheck, F., Wittmer, C., Clauditz, T.S., Krech, T., Tsourlakis, M.C., Minner, S., Graefen, M., Schlomm, T., Wilczak, W. and Jacobsen, F. (2017): High-Level Glyoxalase 1 (GLO1) expression is linked to poor prognosis in prostate cancer. Prostate, 77, 1528-1538.
- Cameron, A.D., Olin, B., Ridderström, M., Mannervik, B. and Jones, T.A. (1997): Crystal structure of human glyoxalase I--evidence for gene duplication and 3D domain swapping. EMBO J., 16, 3386-3395.
- Cheng, W.L., Tsai, M.M., Tsai, C.Y., Huang, Y.H., Chen, C.Y., Chi, H.C., Tseng, Y.H., Chao, I.W., Lin, W.C., Wu, S.M., Liang, Y., Liao, C.J., Lin, Y.H., Chung, I.H., Chen, W.J., Lin, P.Y., Wang, C.S. and Lin, K.H. (2012): Glyoxalase-I is a novel prognosis factor associated with gastric cancer progression. PLoS One, 7, e34352.
- Hikita, K., Yamada, S., Shibata, R., Katoh, M., Murata, T., Kato, K., Tanaka, H. and Kaneda, N. (2015): Inhibitory effect of isoflavones from Erythrina poeppigiana on the growth of HL-60 human leukemia cells through inhibition of glyoxalase I. Nat. Prod. Commun., 10, 1581-1584.
- Islam, A., Rodrigues, B.L., Marzano, I.M., Perreira-Maia, E.C., Dittz, D., Paz Lopes, M.T., Ishfaq, M., Frézard, F. and Demicheli, C. (2016): Cytotoxicity and apoptotic activity of novel organobismuth(V) and organoantimony(V) complexes in different cancer cell lines. Eur. J. Med. Chem., 109, 254-267.
- Iuchi, K., Hatano, Y. and Yagura, T. (2008): Heterocyclic organobismuth(III) induces apoptosis of human promyelocytic leukemic cells through activation of caspases and mitochondrial perturbation. Biochem. Pharmacol., 76, 974-986.
- Iuchi, K., Akagi, K. and Yagura, T. (2009): Heterocyclic organobismuth(III) compound targets tubulin to induce G2/M arrest in HeLa cells. J. Pharmacol. Sci., 109, 573-582.
- Jin, T., Zhao, L., Wang, H.P., Huang, M.L., Yue, Y., Lu, C. and Zheng, Z.B. (2020): Recent advances in the discovery and development of glyoxalase I inhibitors. Bioorg. Med. Chem., 28, 115243.
- Kamepalli, S., Carmalt, C.J., Culp, R.D., Cowley, A.H., Jones, R.A. and Norman, N.C. (1996): Synthesis and structures of intramolecularly base-coordinated aryl group 15 compounds. Inorg. Chem., 35, 6179-6183.
- Kang, Y., Edwards, L.G. and Thornalley, P.J. (1996): Effect of methylglyoxal on human leukaemia 60 cell growth: modification of DNA G1 growth arrest and induction of apoptosis. Leuk. Res., 20, 397-405.
- Kohri, K., Yoshida, E., Yasuike, S., Fujie, T., Yamamoto, C. and Kaji, T. (2015): The cytotoxicity of organobismuth compounds with certain molecular structures can be diminished by replacing the bismuth atom with an antimony atom in the molecules. J. Toxicol. Sci., 40, 321-327.
- Lee, J.H., Subedi, L. and Kim, S.Y. (2020): Effect of cysteine on methylglyoxal-induced renal damage in mesangial cells. Cells, 9, 234.
- Liu, Y.P., Lei, J., Tang, L.W., Peng, Y., Au, C.T., Chen, Y. and Yin, S.F. (2017): Studies on the cytotoxicity and anticancer performance of heterocyclic hypervalent organobismuth(III) compounds. Eur. J. Med. Chem., 139, 826-835.
- Murafuji, T., Azuma, T., Miyoshi, Y., Ishibashi, M., Rahman, A.F., Migita, K., Sugihara, Y. and Mikata, Y. (2006): Inhibition of jack bean urease by organobismuth compounds. Bioorg. Med. Chem. Lett., 16, 1510-1513.
- Ohtaka, K., Takemoto, S., Ohnishi, M. and Akiba, K. (1989): Synthesis and chemical behaviors of 12-substituted dibenz[c,f][1,5]azastibocine and dibenz[c,f][1,5]azabismocine derivatives: evidences of 10-Pn-4 type hypervalent interaction. Tetrahedron Lett., 30, 4841-4844.
- Onishi, K., Douke, M., Nakamura, T., Ochiai, Y., Kakusawa, N., Yasuike, S., Kurita, J., Yamamoto, C., Kawahata, M., Yamaguchi, K. and Yagura, T. (2012): A novel organobismuth compound, 1-[(2-di-p-tolylbismuthanophenyl)diazenyl]pyrrolidine, induces apoptosis in the human acute promyelocytic leukemia cell line NB4 via reactive oxygen species. J. Inorg. Biochem., 117, 77-84.
- Ranganathan, S. and Tew, K.D. (1993): Analysis of glyoxalase-I from normal and tumor tissue from human colon. Biochim. Biophys. Acta, 1182, 311-316.
- Sakamoto, H., Mashima, T., Kizaki, A., Dan, S., Hashimoto, Y., Naito, M. and Tsuruo, T. (2000): Glyoxalase I is involved in resistance of human leukemia cells to antitumor agent-induced apoptosis. Blood, 95, 3214-3218.
- Sakamoto, H., Mashima, T., Sato, S., Hashimoto, Y., Yamori, T. and Tsuruo, T. (2001): Selective activation of apoptosis program by S-p-bromobenzylglutathione cyclopentyl diester in glyoxalase I-overexpressing human lung cancer cells. Clin. Cancer Res., 7, 2513-2518.
- Santarius, T., Bignell, G.R., Greenman, C.D., Widaa, S., Chen, L., Mahoney, C.L., Butler, A., Edkins, S., Waris, S., Thornalley, P.J., Futreal, P.A. and Stratton, M.R. (2010): GLO1-A novel amplified gene in human cancer. Genes Chromosomes Cancer, 49, 711-725.
- Takasawa, R., Takahashi, S., Saeki, K., Sunaga, S., Yoshimori, A. and Tanuma, S. (2008): Structure-activity relationship of human GLO I inhibitory natural flavonoids and their growth inhibitory effects. Bioorg. Med. Chem., 16, 3969-3975.
- Takasawa, R., Saeki, K., Tao, A., Yoshimori, A., Uchiro, H., Fujiwara, M. and Tanuma, S. (2010): Delphinidin, a dietary anthocyanidin in berry fruits, inhibits human glyoxalase I. Bioorg. Med. Chem., 18, 7029-7033.
- Takasawa, R., Tao, A., Saeki, K., Shionozaki, N., Tanaka, R., Uchiro, H., Takahashi, S., Yoshimori, A. and Tanuma, S. (2011): Discovery of a new type inhibitor of human glyoxalase I by myricetin-based 4-point pharmacophore. Bioorg. Med. Chem. Lett., 21, 4337-4342.
- Takasawa, R., Shimada, N., Uchiro, H., Takahashi, S., Yoshimori, A. and Tanuma, S. (2016): TLSC702, a novel inhibitor of human glyoxalase I, induces apoptosis in tumor cells. Biol. Pharm. Bull., 39, 869-873.
- Takasawa, R., Akahane, H., Tanaka, H., Shimada, N., Yamamoto, T., Uchida-Maruki, H., Sai, M., Yoshimori, A. and Tanuma, S.I. (2017): Piceatannol, a natural trans-stilbene compound, inhibits human glyoxalase I. Bioorg. Med. Chem. Lett., 27, 1169-1174.
- Tamori, S., Nozaki, Y., Motomura, H., Nakane, H., Katayama, R., Onaga, C., Kikuchi, E., Shimada, N., Suzuki, Y., Noike, M., Hara, Y., Sato, K., Sato, T., Yamamoto, K., Hanawa, T., Imai, M., Abe, R., Yoshimori, A., Takasawa, R., Tanuma, S.I. and Akimoto, K. (2018): Glyoxalase 1 gene is highly expressed in basal-like human breast cancers and contributes to survival of ALDH1-positive breast cancer stem cells. Oncotarget, 9, 36515-36529.
- Thornalley, P.J. (1990): The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem. J., 269, 1-11.
- Thornalley, P.J., Edwards, L.G., Kang, Y., Wyatt, C., Davies, N., Ladan, M.J. and Double, J. (1996): Antitumour activity of S-p-bromobenzylglutathione cyclopentyl diester in vitro and in vivo. Inhibition of glyoxalase I and induction of apoptosis. Biochem. Pharmacol., 51, 1365-1372.
- Wang, Y., Kuramitsu, Y., Ueno, T., Suzuki, N., Yoshino, S., Iizuka, N., Akada, J., Kitagawa, T., Oka, M. and Nakamura, K. (2012): Glyoxalase I (GLO1) is up-regulated in pancreatic cancerous tissues compared with related non-cancerous tissues. Anticancer Res., 32, 3219-3222.
- Zhang, L., Mulrooney, S.B., Leung, A.F., Zeng, Y., Ko, B.B., Hausinger, R.P. and Sun, H. (2006): Inhibition of urease by bismuth(III): implications for the mechanism of action of bismuth drugs. Biometals, 19, 503-511.
- Zhitkovich, A. (2019): N-Acetylcysteine: antioxidant, aldehyde scavenger, and more. Chem. Res. Toxicol., 32, 1318-1319.